Vietnam begins Covid-19 vaccine human trial
This is the first phase of the Vietnam-made vaccine clinical trial program.
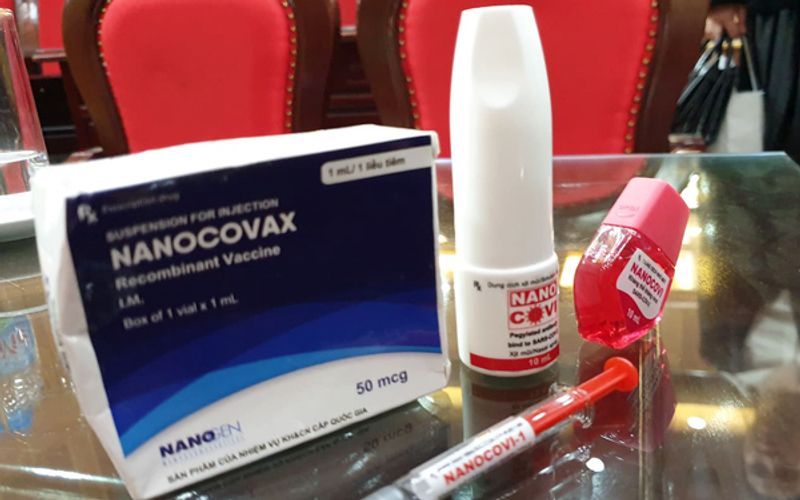
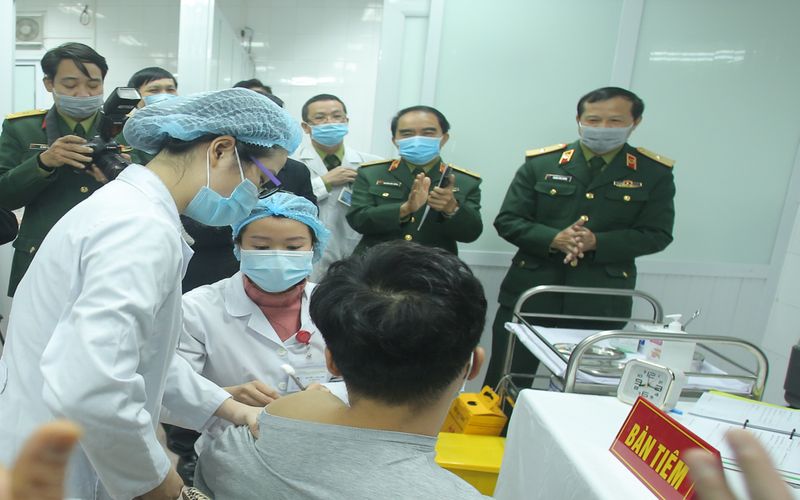
Vietnam started Covid-19 vaccine human trial on 60 volunteers who were injected with Nanocovax, the first made-in-Vietnam Covid-19 vaccine approved for human trials, on December 17.
This is the first phase of the Vietnam-made vaccine clinical trial program, said Nguyen Ngo Quang, deputy head of the Administration of Science, Technology and Training under the Ministry of Health.
Mr. Quang confirmed around 200 people had signed up within a week to be vaccinated with Nanocovax, a Covid-19 vaccine developed by Nanogen Pharmaceutical Biotechnology JSC.
Vietnam Military Medical Academy, as part of first phase trials, has been overseeing administration of the Covid-19 vaccine.
Nanogen's two vaccine products include injectable and spray form. |
The academy has been racing against time to screen eligible volunteers for the first human trial stage. Selected candidates are between 18 and 50 years of age.
They will receive two intramuscular shots of the vaccine, with an interval of 28 days between them. The volunteers will have their health monitored for 56 days to assess the efficiency of the vaccine and continue to be under observation for sixth months following administration.
Dr. Ngo Quang also said that all the volunteers have been covered by health insurance. The second and third trial phases will be conducted in March 2021 and August 2021 on 3,000-4,000 people or even 10,000.
According to Professor Do Quyet, director of the Vietnam Military Medical Academy, his institution and Nanogen have prepared very well for the clinical trial stages. In particular, the volunteers’ safety will be the top priority.
He noted that the strength of Vietnam-made Covid-19 vaccines would be normal refrigerator temperature (2-8 degrees Celsius) storage, while vaccines of some foreign manufacturers must be stored in minus 75 degrees Celsius, which will result of a lot of transport inconvenience.
Do Minh Si, Nanogen's director for research and development, said all risks and variables have been assessed, with medical staff from 103 Military Hospital and the Vietnam National Institute of Burns on standby.
Nanogen has signed contracts with an insurance company to cover the unexpected cases as well as allocated a funding of up to VND20 billion (US$863,290) to pay for any accident not covered by insurance, Mr. Si stressed.
Nanocovax is expected to be priced at VND120,000 (US$5.17) per dose, the Nanogen's director said, adding that the vaccine would be reasonably priced and affordable to all Vietnamese, and hopefully included in health insurance policy. The vaccine is scheduled to enter mass production in May 2021.
Vietnam currently has four Covid-19 vaccines under development, by Nanogen, the Institute of Vaccines and Medical Biologicals (IVAC), the Vaccine and Biological Production Company No. 1 (Vabiotech) and the Center for Research and Production of Vaccines and Biologicals (Polyvac).
The Covid-19 vaccine developed by IVAC is expected to enter human trials in March next year if approved by authorities. Vabiotech said it would seek approval for human trials in early 2021.
Vietnam has recorded 1,405 Covid-19 cases so far, 115 still active, most of whom are imported cases. Thirty-five have succumbed to the disease, many being elderly patients with underlying conditions like diabetes or kidney failure. No community transmission has been recorded in two weeks.
Clinical room for volunteers. |
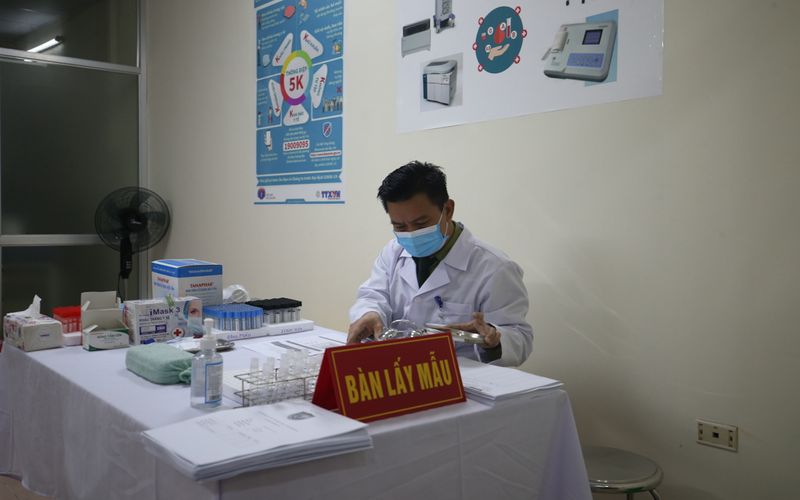
Sample-taking table for volunteers. |
Clinical table for volunteers. |
Room for 72-hour supervision after volunteers are taken samples. |
The first volunteer was vaccinated with Nanocovax on December 17. |