Japan’s drug maker to begin Covid-19 vaccine Phase 3 clinical trial in Vietnam
The clinical trial is aimed at putting its "recombinant protein vaccine" on the list of the World Health Organization-approved vaccines.
Japanese drugmaker Shionogi will begin a Phase 3 clinical trial of its Covid-19 vaccine this month, mainly in Vietnam and other Asian countries, Nikkei Asia cited Shionogi CEO Isao Teshirogi as saying.
CEO Isao Teshirogi said that the clinical trial, which will involve two groups, one given the actual vaccine and the other a placebo, is aimed at putting its "recombinant protein vaccine" on the list of the World Health Organization-approved vaccines.
The vaccine would utilize "genetically modified insect cells" to produce copies of the coronavirus spike protein to trigger an immune response, a technique that has been used by several other vaccine manufacturers.
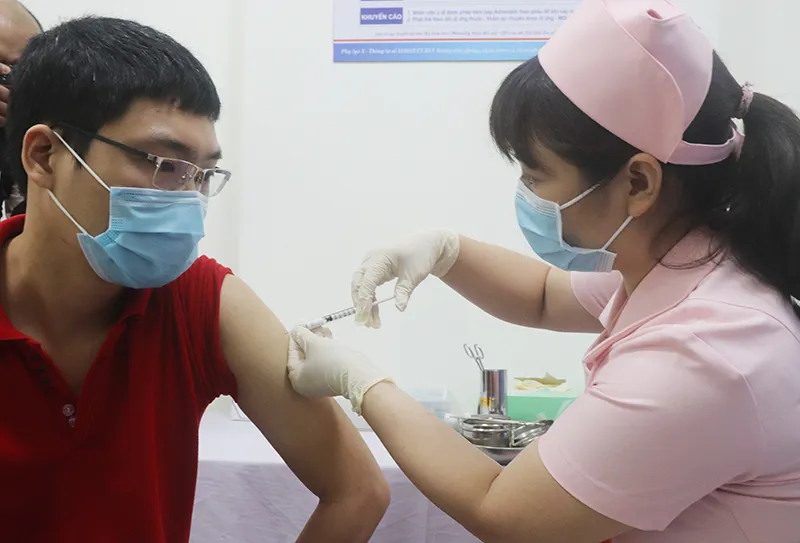
A volunteer joins a clinical trial of Covid-19 vaccine in Vietnam. Photo: Thao Tran/The Hanoi Times |
Shionogi said the firm is seeking approval for the vaccine in Japan to make it available domestically by the end of March 2022. The firm is the front-runner in Covid-19 vaccine development in Japan. Vietnam has agreed to hold a trial in exchange for technology transfer, Teshirogi added.
The placebo-based trial is one of several Phase 3 clinical trials the Osaka-based company is planning to undertake. In Japan, the company will also examine the safety and efficacy of its vaccine compared with those of Pfizer, Moderna, and AstraZeneca, which are currently being administered. It will also test the efficacy of the vaccine as a booster shot.
Shionogi hopes to obtain approval from Japanese regulators and make the vaccine available in Japan by the end of March next year, Teshirogi said.
He stressed that Shionogi is also developing a Covid-19 drug whose clinical trials have already begun in Japan since September. Vietnam, along with Singapore, South Korea, and the UK, is also expected to begin trial for the drug as early as this month. More than 90% of the test cases may be from overseas, as there are fewer than 300 new Covid-19 carriers detected in Japan a day.
Teshirogi noted that the trial in Singapore, where 3,000 to 4,000 new cases are reported daily despite more than 80% of its population being fully vaccinated, would provide useful insights for Japan, which also has more than 70% of its population fully vaccinated.
Previously in July, Shionogi had signed a contract with Vietnam's Vabiotech to transfer Covid-19 vaccine production technologies, according to the Ministry of Health.
Japan is one of Vietnam’s leading vaccine donors with 4.08 million doses of the AstraZeneca vaccine delivered to the Southeast Asian nation so far.
Besides vaccines, Japan has also provided Vietnam with financial aid to purchase medical supplies for the Covid-19 fight. Japanese businesses operating in Vietnam have raised funds to support the fight in the country.
Vietnam has vaccinated around 60 million people with at least one Covid-19 vaccine shot and over 25.1 million people have been fully vaccinated.